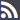List of elements which should at least be included in prescriptions issued in a Member State to be recognised in another Member State
This Directive lays down measures for the uniform implementation of Article 11(1) of Directive 2011/24/EU concerning the recognition of medical prescriptions issued in another Member State.
The DIRECTIVE 2012/52/EU
 of 20 December 2012 have approved the least of minium elements required in medical prescriptions issued in another Member State. The list of elements included in medical prescriptions are following listed:
of 20 December 2012 have approved the least of minium elements required in medical prescriptions issued in another Member State. The list of elements included in medical prescriptions are following listed:
Non-exhaustive
Headings appearing in bold in this Annex are not required to feature in prescriptions
Identification of the patient
Surname(s)
First name(s) (written out in full, i.e. no initials)
Date of Birth
Authentication of the prescription
Issue date
Identification of the prescribing health professional
Surname(s)
First name(s) (written out in full, i.e. no initials)
Professional qualification
Details for direct contact (email and telephone or fax, the latter both with international prefix)
Work address (including the name of the relevant Member State)
Signature (written or digital, depending on the medium chosen for issuing the prescription)
Identification of the prescribed product, where applicable
‘Common name’ as defined by Article 1 of Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use
The brand name if:
| (a) | the prescribed product is a biological medicinal product, as defined in point 3.2.1.1.(b) of Annex I (Part I) to Directive 2001/83; or |
| (b) | the prescribing health professional deems it medically necessary; in that case the prescription shall shortly state the reasons justifying the use of the brand name |
Pharmaceutical formulation (tablet, solution, etc.)
Quantity
Strength, as defined in Article 1 of Directive 2001/83/EC
Dosage regimen
 Puntos nacionales de contacto de la UE Anexo : Criterios básicos de las Recetas Médicas
Puntos nacionales de contacto de la UE Anexo : Criterios básicos de las Recetas Médicas
If you want to find any information related to this website, please use the search application on the top of the page