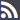Royal Decree 81/2014 of 7 February establishing rules to ensure cross-border healthcare and amending Royal Decree 1718/2010 of 17 December on medical prescriptions and dispensing orders.
Please notice that this is not a certified translation of the Spanish Royal decree 81/2014.
Published in: «BOE» No. 34, February 8, 2014, pp. 10915-10948 (34 p.)
Section: I. General Provisions
Department: Ministry of Health, Social Services and Equality
Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients' rights in cross-border healthcare is aimed at ensuring patient mobility, establishing some rules for facilitating access to safe and high-quality healthcare and promoting cooperation on healthcare between Member States, whilst fully respecting their responsibilities in the organisation and delivery of such healthcare. As soon as the corresponding legal instruments are implemented, Directive 2011/24/EU shall likewise be applied to the States forming part of the European Economic Area.
The Directive shall apply to individual patients who decide to seek healthcare in a Member State other than their Member State of affiliation.
The legal grounds on which it is based are twofold. On the one hand, Article 168 of the Treaty on the Functioning of the European Union (TFEU), according to which a high level of human health protection shall be ensured and, on the other, Article 114 of the TFEU on the improvement of the functioning of the internal market and the free movement of goods, persons and services. Hence, in its preamble it is highlighted that the European Union's health systems, which are an essential component of the highest level of social protection, also form part of the wider framework of general interest services. Healthcare is therefore not excluded from the scope of the fundamental principle of the freedom to provide services.
The spirit of the Directive is to ensure patient mobility and, in order to do so, it makes patients' freedom of choice possible and opposes any kind of discrimination on grounds of nationality. All of the above is in line with considering healthcare as a service that should always guarantee a high level of human health protection.
An essential feature of the Directive is that its requirements do not involve either a new regulation of health systems or a substantial change in them. Nonetheless, a new healthcare scenario in the European Union is being broached as of this moment. It proclaims full respect for the differences in national health systems and the Member States' responsibilities concerning the organisation and delivery of healthcare services, recognising the freedom to structure their own health and social security systems.
The Directive also aims to clarify the relationships of the cross-border healthcare it regulates with the existing coordination framework for social security systems contained in Regulation (EC) No 883/2004 of the European Parliament and of the Council of 29 April 2004 on the coordination of social security systems and in Regulation (EC) No 987/2009 of the European Parliament and of the Council of 16 September 2009 laying down the procedure for implementing Regulation (EC) No 883/2004 on the coordination of social security systems.
Both instruments broadly coincide in their subjective and objective areas of application. The most notable difference is that, according to the Directive, patients will pay for the healthcare received in advance, which shall subsequently be reimbursed depending on the cases, whereas such general obligation does not exist in the regulations. Another significant difference is that the Directive applies to all healthcare providers, regardless of whether they are public or private providers, while the regulations only coordinate social security systems.
Along with the guarantees on information, access to safe and high-quality healthcare and cooperation on healthcare matters among Member States, the main thrust of the Directive lies in regulating the reimbursement of costs and prior authorisation for insured persons. These notions had already been regulated by Regulation (EC) No 883/2004 of 29 April 2004, but with a very different scope and approach.
The assumption of costs arising from cross-border healthcare by the States obliged to do so is limited to the amount a State would have covered had the healthcare been provided on its own territory, without exceeding the real costs of the healthcare actually delivered. These reimbursement costs shall be set through a transparent calculation procedure based on objective non-discriminatory criteria which are known in advance.
The rules for reimbursement may only be limited on grounds of overriding reasons of general interest, which is to be restricted to measures that are necessary and proportionate, without becoming a means of random discrimination or an unjustified obstacle to the free movement of persons, goods and services.
In Spain, people insured in accordance with Spanish legislation and their beneficiaries, as well as people regarding which Spain holds the competence to grant the necessary prior authorisation for planned treatment according to EU social security regulations are entitled to the reimbursement of costs arising from cross-border healthcare.
The Spanish State, as a Member State of affiliation, guarantees the reimbursement of costs to which an insured person who has received cross-border healthcare is entitled. The competent health authority in Spain shall be obliged to assume such reimbursement. With regard to the regulation of the reimbursement of costs and prior authorisation, the option chosen in this Royal Decree is the most coherent with our National Health Service.
The reimbursement of costs is limited to the healthcare set forth in the National Health Service's common portfolio of services and, as appropriate, in the complementary portfolio of the relevant regional authority. As is logical, the same conditions and steps are required that would have been imposed had the healthcare been delivered on Spanish territory for the corresponding healthcare services assigned.
The freedom to choose the cross-border healthcare option is only limited in a small number of healthcare benefits, which are the ones subject to prior authorisation, among the range of possible benefits available. This presupposes a mechanism which, aside from allowing proper planning by the competent health authority, offers guarantees to patients to ensure they know in advance if the healthcare treatment and the conditions under which it is sought will be included in the portfolio of services so that it may be reimbursed.
The Spanish State, as a Member State of treatment, holds responsibility for and has to promote certain quality and safety levels in healthcare. It should also ensure the existence of complaints and redress mechanisms for any possible damages arising from the healthcare received and the transfer of information to ensure continuity of healthcare for both its own citizens as well as for EU citizens in general.
This is complemented by an extensive and highly transparent information policy, which is materialised through the setting up of the so-called "national contact point", given that patients need adequate information on the essential aspects of cross-border healthcare in order to exercise their right to such healthcare in practice.
With regard to the transfer of information on health professionals, information on such professionals' right to exercise their profession and on their suspension or disqualification thereof should be guaranteed.
The Directive also includes a section dedicated to cooperation on healthcare, which includes the recognition of prescriptions, European Reference Networks, rare diseases, eHealth and the assessment of health technologies.
In Spain, Article 43 of the Constitution provides the basic legal framework on health which has allowed an organisational model to be established that guarantees quality healthcare and makes effective a catalogue of patients' rights. Act 14/1986 of 25 April, known as the General Health Act, Act 41/2002 of 14 November governing patient autonomy and the rights and obligations concerning clinical information and documentation, Act 44/2003 of 21 November regulating health professions and Act 16/2003 of 28 May on the cohesion and quality of the National Health Service all respond to this framework. The catalogue of rights of the National Health Service's users are set forth in these pieces of legislation and in their implementing provisions, including matters having to do with patient safety and the quality of healthcare.
To sum up, the new framework set forth by the Directive involves an increase in quality and security guarantees for patient-users, but also the sector's liberalisation. The latter will constitute an opportunity for private healthcare and a challenge for the public health sector, which will in any event have to respect the essential values of universality, access to high-quality healthcare, equity and solidarity for patients and citizens regardless of their Member State of affiliation. The health services of the regional authorities will, on the one hand, have to deal with a possible increase in the number of patients from other Member States and, on the other, they will have to reimburse the costs of their affiliates who have decided to receive healthcare in another EU Member State.
This Royal Decree is structured around twenty-four articles that are divided into six chapters, seven additional provisions, a transitional provision, five final provisions and two annexes.
Chapter I sets forth the general provisions, subject matter and scope of application – from which long-term care, the allocation and transplant of organs and public vaccination programmes have been excluded, without detriment to cooperation between Spain and other Member States on these matters –, as well as the definitions which apply.
Chapter II lays down the guarantees to gain access to safe and high-quality cross-border healthcare for citizens whose Member State of affiliation is Spain seeking healthcare in another State of the European Union as well as for patients whose State of affiliation is another Member State seeking healthcare from public or private providers in our country through mechanisms like information, claims to seek redress for any possible damages, continuity of treatment and the protection of privacy with regard to treatment.
Chapter III refers to the information to be provided, which must be accessible and up to date, by the national contact point that is set up and regulated in order to ensure information is provided to patients whose Member State of affiliation is Spain and to patients with another Member State of affiliation who seek to receive to healthcare in Spain. It also refers to the information to be provided by healthcare providers in our country on their offering of services, accreditation, quality and safety, availability, prices and the necessary guarantees to cover liability for any possible damages.
Chapter IV sets forth the provisions on the reimbursement of costs arising from cross-border healthcare, including the general principles for the reimbursement of costs, the reimbursement rates which apply and the procedure laid down to make reimbursements.
Chapter V is dedicated to the healthcare requiring prior authorisation, including the application procedure and the reasons for its refusal.
Chapter VI refers to cooperation on healthcare between Spain and other Member States in several areas, including: information on health professionals, recognition of prescriptions issued in another Member State, European reference networks, rare diseases, eHealth and health technology assessment.
Concerning information transfer on health professionals, the agency of the Ministry of Health, Social Services and Equality in charge of organising and managing the National Registry of Health Professionals, the regional authorities' departments of health and the governing boards of health professional associations have been assigned with the responsibility to inform about health professionals' right to exercise their profession and on their suspension or their disqualification thereof within the scope of their competencies; in the latter case, only where an act lays down the obligation of joining a professional association to exercise professional activities or healthcare professions. All the above will be done through the Ministry of Health, Social Services and Equality to ensure coordination of any information sent and that it coincides with the National Registry of Health Professionals.
The first final provision amends certain provisions of Royal Decree 1718/2010 of 17 December on medical prescriptions and dispensing orders to make them comply with the provisions contained in Directive 2011/24/EU of 9 March 2011 and in Implementing Directive 2012/52/EU of the Commission of 20 December 2012 laying down measures to facilitate the recognition of medical prescriptions issued in another Member State.
Lastly, the annexes reflect the documentation required for the reimbursement procedure and the healthcare benefits subject to prior authorisation.
This Royal Decree transposes into the internal Spanish legal system Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients' rights in cross-border healthcare and Implementing Directive 2012/52/EU of the Commission of 20 December 2012 laying down measures to facilitate the recognition of medical prescriptions issued in another Member State.
The regional authorities and the cities of Ceuta and Melilla have been consulted in the process of drafting this legislation, which was submitted for its approval to a plenary session of the Interterritorial Council of the National Health Service and its consultative committee. The sectors affected by it have been given a hearing and consultations have been held with the administrative mutual societies for the State's civil servants, the armed forces and the personnel at the service of the justice administration, and it was submitted for a report from the Spanish Data Protection Agency. The Interministerial Commission for the study of matters having a significant budgetary impact on the National Health Service's financial balance or significant economic implications has likewise been consulted.
This Royal Decree is enacted pursuant to the provisions contained in Article 149.1(16) of the Constitution, which grants the State exclusive competence on the rules and overall coordination of health. The amendment of Royal Decree 1718/2010 of 17 December on medical prescriptions and dispensing orders, which is done through the first final provision, is excluded from the above and grounded on the State's competence to enact legislation on pharmaceutical products.
By virtue thereof, at the proposal of the Minister of Health, Social Services and Equality and with the prior approval of the Minister of Finance and Public Administrations and the agreement of the Council of State and after its deliberation by the Cabinet at its meeting held on 7 February 2014,
I HEREBY DECREE:
CHAPTER I
General provisions
Article 1. Subject matter.
The aim of this Royal Decree is to lay down the rules to facilitate access to safe and high-quality cross-border healthcare and to promote cooperation on healthcare between Spain and other Member States of the European Union.
This legislation does not affect the patients' rights set forth in Regulation (EC) No 883/2004 of 29 April 2004 on the coordination of social security systems or in Regulation (EC) 987/2009 of 16 September 2009 laying down the procedure for implementing Regulation (EC) No 883/2004. This Royal Decree shall not apply where the provisions on cross-border healthcare set forth in said Regulations apply, unless the patient expressly requests its application.
Article 2. Scope of application.
1. This Royal Decree shall apply to the provision of cross-border healthcare to patients, as defined in Article 3, regardless of how it is organised, delivered and funded.
2. The following shall be excluded from its scope of application:
a) Services in the field of long-term care, the purpose of which is to support people in need of assistance in carrying out routine, everyday tasks;
b) The allocation of and access to organs for the purpose of organ transplants;
c) Public vaccination programmes against infectious diseases which are exclusively aimed at protecting the health of the population in Spanish territory and which are subject to specific planning and execution measures, without prejudice to cooperation between Spain and other Member States of the European Union.
3. The provisions contained in this Royal Decree shall not affect the provisions on the organisation and funding of healthcare in situations not related to cross-border healthcare.
In particular, none of the provisions of this Royal Decree shall oblige the reimbursement to patients of costs arising from the healthcare provided to them by healthcare providers established in Spanish territory that are beyond the legal planning and entitlement framework of the National Health Service.
Article 3. Definitions.
For the purpose of this Royal Decree:
1. "Healthcare" means health services provided by health professionals to patients to assess, maintain or restore their state of health, including the prescription, dispensing and provision of medicinal products, medical devices and dietetic foods meant for special medicinal uses.
2. "Cross-border healthcare" means healthcare provided or prescribed in a Member State other than the Member State of affiliation.
3. "Insured person" means:
a) According to Article 2 of Regulation (EC) No 883/2004 of the European Parliament and of the Council of 29 April 2004 on the coordination of social security systems, nationals of a Member State, stateless persons and refugees residing in a Member State who are or have been subject to the legislation of one or more Member States, as well as the members of their families and their survivors, irrespective of the nationality of such persons, where such survivors are nationals of one of the Member States or stateless persons or refugees residing in one of the Member States.
All of the above shall apply, provided the conditions required by the legislation of the competent Member State are met according to Title II of Regulation (EC) No 883/2004 of 29 April 2004.
b) Nationals of a third country covered by Council Regulation (EC) No 859/2003 of 14 May 2003 extending the provisions of Regulation (EEC) No 1408/71 and Regulation No (EEC) 574/72 to nationals of third countries who are not already covered by those provisions solely on the ground of their nationality, or by Regulation (EU) No 1231/2010 of the European Parliament and of the Council of 24 November 2010 extending Regulation (EC) No 883/2004 and Regulation (EC) No 987/2009 to nationals of third countries who are not already covered by those provisions solely on the ground of their nationality, or who meet the conditions laid down in the legislation of the Member State of affiliation to have entitlement to the benefits.
4. "Member State of affiliation" means:
a) For persons referred to in point 3(a), the Member State that is competent to grant to the insured person a prior authorisation to receive appropriate treatment outside the Member State of residence according to Regulation (EC) No 883/2004 of 29 April 2004 and Regulation (EC) No 987/2009 of 16 September 2009.
b) For persons referred to in point 3(b), the Member State that is competent to grant to the insured person a prior authorisation to receive appropriate treatment in another Member State according to Regulation (EC) No 859/2003 of 14 May 2003 or Regulation (EU) No 1231/2010 of 24 November 2010. If no Member State is competent according to those Regulations, the Member State of affiliation shall be the Member State where the person is insured or has the rights to sickness benefits according to the legislation of that Member State.
5. "Member State of treatment" means the Member State on whose territory healthcare is actually provided to the patient. In the case of telemedicine, healthcare is considered to be provided in the Member State where the healthcare provider is established.
6. "Competent health authority" means the public health authority in charge of providing healthcare to the insured person and, in terms of the public funding of medicinal products and medical devices included among the pharmaceutical benefits of the National Health Service, the Ministry of Health, Social Services and Equality.
7. "Health professional" means any person deemed as such by the legislation of the Member State of treatment. In the case of Spain, any person who is legally entitled to exercise a qualified and regulated health profession in accordance with provisions set forth in Act 44/2003 of 21 November regulating health professions and in the seventh additional provision of Act 33/2011 of 4 October, the General Health Act.
8. "Healthcare provider" means any natural or legal person that legally provides healthcare on the territory of a Member State. In the case of Spain, the health centres, services or units and healthcare establishments authorised, catalogued and registered according to Royal Decree 1277/2003 of 10 October laying down the general rules on the authorisation of health centres, services and establishments.
9. "Patient" means any natural person who seeks to receive or receives healthcare in a Member State.
10. "Medicinal product" means, pursuant to Article 8 of Act 29/2006 of 26 July on guarantees for and rational use of medicinal products and medical devices, any substance or combination of substances that is presented as having properties for the treatment or prevention of diseases in human beings, or which may be used or administered to human beings with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis.
11. "Medical device" means, pursuant to Article 8 of Act 29/2006 of 26 July on guarantees for and rational use of medicinal products and medical devices, any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, together with any accessories, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of:
1.- diagnosis, prevention, control, treatment or alleviation of a disease;
2.- diagnosis, control, treatment, alleviation or compensation of an injury or handicap;
3.- investigation, replacement or modification of the anatomy or of a physiological process;
4.- control of conception;
and which does not achieve its principal or intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
12. "Prescription" means the document on which a treatment with a medicinal product or a medical device is prescribed issued by a member of a regulated health profession who is legally entitled to do so in the Member State in which the prescription is issued. In the case of Spain, a prescription issued by a person who exercises a regulated health profession where legally empowered to do so according to the provisions set forth in Article 4 of Royal Decree 1837/2008 of 8 November transposing into the Spanish legal system Directive 2005/36/EC of the European Parliament and of the Council of 7 September 2005 and Directive 2006/100/EC of the Council of 20 November 2006 on the recognition of professional qualifications, as well as on certain aspects of exercising the profession of lawyer.
13. "Health technologies" means a medicinal product, a medical device or medical and surgical procedures as well as measures for disease prevention, diagnosis or treatment used in healthcare.
14. "Health record or medical record" means all the documents containing data, assessments and information of any kind on a patient’s situation and clinical development throughout the care process, regardless of their format. In the case of Spain, medical records are governed by the provisions set forth in Act 41/2002 of 14 November, basic Act governing patient autonomy and the rights and obligations concerning clinical information and documentation.
Article 4. General principles of cross-border healthcare.
Cross-border healthcare shall be provided in accordance with the legislation of the Member State of treatment, including quality and safety standards and guidelines, and with European Union legislation on safety standards, taking into account the principles of universality, access to good quality care, equity and solidarity.
Where the healthcare is provided in Spain to patients whose State of affiliation is another Member State, the same principle of non-discrimination on grounds of nationality shall apply.
CHAPTER II
Guarantees with regard to cross-border healthcare
Article 5. Healthcare delivered in another Member State to patients whose State of affiliation is Spain.
1. The costs arising from healthcare delivered in another Member State to a patient whose State of affiliation is Spain shall be paid by the patient and their reimbursement shall be effectuated in accordance with the conditions and requirements set forth herein, provided Regulation (EC) No 883/2004 of 29 April 2004 does not apply, or even if it applies, should the patient request it.
2. The national contact point regulated in Article 7 shall provide the necessary information so that patients may exercise their rights to cross-border healthcare. Patients may also direct their queries to the national contact point of the Member State of treatment. Information on the rights arising from Regulation (EC) No 883/2004 of 29 April 2004 shall likewise be provided.
3. With a view to facilitating continuity of healthcare, the following shall be guaranteed to patients receiving healthcare in another Member State:
a) Availability of a copy of their clinical reports on appropriate media, along with the results of diagnostic tests and/or therapeutic procedures, providing information on the procedure to gain access to them. Public administrations shall promote access to clinical documentation by means of the information systems laid down for such purpose by the legal system.
b) Medical follow-up in Spain after the healthcare is delivered, in the same way as if the care received in another Member State had been delivered in Spain.
c) Cooperation on timely information transfer with other Member States to ensure continuity of healthcare. In this regard, Article 23 on matters connected to eHealth shall be taken into account. In the aforementioned information transfer, Spain shall apply national, European and international standards on the transfer of electronic medical records or their components.
d) The security guarantees on data processing laid down in Spanish personal data protection legislation.
Article 6. Healthcare delivered in Spain to patients whose State of affiliation is another Member State
Without prejudice to the rights conferred by Regulation (EC) No 883/2004 of 29 April 2004 and Regulation (EC) No 987/2009 of 16 September 2009, patients whose State of affiliation is another Member State shall have the following guarantees with regard to the healthcare delivered to them in Spain pursuant to this Royal Decree:
1. The necessary information shall be provided to exercise the right to cross-border healthcare through the Spanish national contact point and healthcare providers.
2. Access shall be provided to the complaints procedures and mechanisms laid down to seek redress in accordance with the Spanish legal system for damages resulting from the healthcare received.
3. In order to facilitate continuity of healthcare, the following shall be guaranteed:
a) Availability of a copy of their clinical reports on appropriate media, along with the results of diagnostic tests and/or therapeutic procedures, providing information on the procedure to gain access to them. Public administrations shall promote access to clinical documentation by means of the information systems laid down for such purpose by the legal system.
b) Cooperation on timely information transfer with other Member States to ensure continuity of healthcare. In this regard, Article 23 on matters connected to eHealth shall be taken into account. In the aforementioned information transfer, Spain shall apply national, European and international standards on the transfer of electronic medical records or their components.
c) Protection of the right to privacy in the processing of personal and health data in accordance with Organic Act 15/1999 of 13 December on the protection of personal data and Act 41/2002 of 14 November, basic Act governing patient autonomy and the rights and obligations concerning clinical information and documentation.
4. The principle of non-discrimination on grounds of nationality shall apply among patients from other Member States and patients whose State of affiliation is Spain.
Notwithstanding the foregoing, the Ministry of Health, Social Services and Equality may, following a report from the Interterritorial Council of the National Health Service, set conditions on citizens whose State of affiliation is another Member State to gain access to treatment in Spain where such conditions are necessary and proportional to ensure sufficient permanent access to the healthcare offered by the regional health services. Furthermore, they shall have to be justified by overriding reasons of general interest, especially due to planning needs aimed at ensuring a balanced range of high- quality treatments or in order to ensure the rational use of financial, technical and human resources. Any measures thus adopted shall be assessed by the European Commission and published by the national contact point, so that they may be known in advance by patients from other States.
CHAPTER III
Information on cross-border healthcare
Article 7. National contact point.
1. A national contact point shall be set up at the Ministry of Health, Social Services and Equality unit holding responsibility for providing information to citizens in order to provide the necessary information on the matters dealt with by this Royal Decree.
2. The National Contact Point shall cooperate with the European Commission and with the national contact points of other Member States. It shall also provide citizens who request them the latter's contact details.
3. The national contact point's activity shall be complemented by the information units of the regional authorities, the National Institute for Health Management and the mutual societies for civil servants within the scope of their competencies.
In addition, the national contact point may collect information from patients’ organisations, professional organisations, private healthcare providers and health insurance bodies and entities.
All the units, organisations and entities mentioned in the two preceding paragraphs shall provide the national contact point with any information requested to properly fulfil its functions.
4. The national contact point shall provide patients whose State of affiliation is Spain and professionals who may request it information on patients' rights to receive cross-border healthcare.
In particular, it shall provide information about the following:
a) Conditions for the reimbursement of costs.
b) Procedures to gain access to cross-border healthcare and to determine its scope and contents, in particular healthcare requiring prior authorisation according to the provisions set forth in Annex II, as well as information on the prior authorisation scheme.
c) Procedures to file complaints or seek redress for damages where the patient considers his rights according to the provisions set forth in this Royal Decree have not been respected.
d) Elements which must be included in prescriptions issued in a Member State and dispensed in another according to the provisions set forth in European legislation.
Information about cross-border healthcare shall clearly differentiate between the rights patients have by virtue of this Royal Decree and the rights ensuing from EU regulations on the coordination of social security systems.
5. The national contact point shall provide information on healthcare in Spain to patients whose State of affiliation is another Member State.
In particular, it shall provide information about the following:
a) Healthcare providers, including, if they request it, information on the entitlement of a specific provider to deliver healthcare and on any possible constraints placed on their practice.
b) Quality and safety standards and guidelines laid down in Spain, including provisions on the supervision and evaluation of healthcare providers, and information on which of them are subject to these standards and guidelines.
c) Information on accessibility to hospitals for disabled persons.
d) Information on patients' rights and on the procedures to file complaints, the mechanisms to seek redress, as well as the judicial and administrative options available to resolve disputes arising from cross-border healthcare according to the provisions set forth in the Spanish legal system.
e) Elements which must be included in prescriptions issued in a Member State and dispensed in another according to the provisions set forth in European legislation.
6. The information referred to in this Article shall be easily accessible and be made available on the website of the Ministry of Health, Social Services and Equality in accessible formats for disabled persons. Such information shall be kept up to date.
Article 8. Information to be provided by healthcare providers.
1. The healthcare provider shall provide the citizen, user or patient information on its location, organisation chart, offering of services, annual activity, the centre's quality indicators, its accreditation or certification regarding quality systems like the specialised healthcare training system, along with information on centres, services and units of reference, the regional authority's accreditation system, quality management systems or certifications with respect of UNE/CEN/ISO standards or others.
2. Information on the healthcare provider's offering of services shall include a description of the organisational characteristics of the services and units, all healthcare procedures and techniques, and the state of the waiting list.
3. The healthcare provider shall provide any clarifications and practical help the user may request on admission procedures and formalities, working hours, documentation or administrative and/or healthcare conditions the patient has to fulfil in order to gain access to the services included in the offering, visiting and accompaniment conditions for the patient and accessibility conditions for disabled persons.
4. The information given by the healthcare provider shall specify the language(s) in which healthcare is provided. Such information shall be given at least in Spanish and the official language of the regional authority in question.
5. The healthcare provider shall visibly display in its access areas information on the kind of centre it is, its name and a list of the units which comprise its authorised healthcare offering, which shall always coincide with the information in the heath authorisation and registry in accordance with Royal Decree 1277/2003 of 10 October and the regional legislation governing this matter.
6. The healthcare provider shall provide the patient with sufficient information to allow him to make a fully informed choice among the treatment options or healthcare alternatives available. The healthcare provider shall also provide, where requested to do so, information on the known outcomes of these options in general medical practice and in the centre itself.
7. The healthcare provider shall guarantee the patient availability of a copy of his medical record to allow for continuity in the delivery of care to patients from other Member States attended by it requiring medical follow-up within the scope of cross-border healthcare.
8. The healthcare provider shall make a list of prices available to citizens in keeping with its offering of services and shall provide clear invoicing information.
Article 9. Healthcare providers' liability.
1. Health professionals who exercise their profession in the private healthcare sector and privately owned legal persons or entities which provide any kind of healthcare services are obliged to have the relevant liability insurance, guarantee or any other kind of financial surety to cover any indemnities that may arise from the provision of such care or services in accordance with the provisions set forth in Article 46 of Act 44/2003 of 21 November regulating health professions.
2. In the area of public healthcare, the liability regime set forth in Title X of Act 30/1992 of 26 November on the Legal Framework of the Public Administrations and on the Common Administrative Procedure shall apply. Nonetheless, each regional authority's health authority may take out and maintain the opportune insurance policies, guarantees or financial surety to cover both the civil liability of the public health service and of its workers, as well as its own financial liability.
3. Healthcare providers shall always be obliged to provide the user of services, when so required by the latter, appropriate information on the coverage of their professional liability insurance or any other means of personal or collective protection they may hold with regard to their professional liability.
CHAPTER IV
Reimbursement of costs arising from cross-border healthcare
Article 10. General principles for reimbursement of costs.
1. Any costs paid for by an insured person whose State of affiliation is Spain who has received cross-border healthcare shall be reimbursed by the corresponding competent health authority according to Article 14, provided such healthcare is included among the benefits the insured person is entitled to in accordance with the National Health Service's common portfolio of services or, as appropriate, according to the provisions set forth in Regulation (EC) No 883/2004 of 29 April 2004.
2. Notwithstanding the foregoing, reimbursement shall be subject to the following exceptions:
a) Persons receiving a pension and their family members residing in Spain if the healthcare is provided by a Member State included in Annex IV of Regulation (EC) No 883/2004 of 29 April 2004 which has recognised such persons' entitlement to healthcare benefits according to the aforementioned Regulation when they are in its territory. In this case, that State shall provide them with healthcare and cover the corresponding costs under its legislation and under the same terms as if they were residing in that Member State.
b) If the healthcare provided is not subject to prior authorisation according to the provisions set forth in this Royal Decree or is not provided in accordance with Regulation (EC) No 883/2004 of 29 April 2004 and is provided on the territory of the Member State which, according to that Regulation, is ultimately responsible for reimbursement of the costs, the costs shall be assumed by that Member State. That Member State may assume the costs of the healthcare in accordance with the terms, conditions, criteria for eligibility and regulatory and administrative formalities that it has established.
3. The costs of the cross-border healthcare shall be reimbursed by the competent health authority up to the amount such authority would have assumed under the same terms and conditions had the healthcare been provided on Spanish territory by the relevant services assigned to such task. Such reimbursement shall be in accordance with the rates approved by the competent health authority, without exceeding the real cost of the healthcare actually delivered and without taking into consideration any related costs.
4. Any insured person who applies for the reimbursement of costs arising from cross-border healthcare shall fulfil the same conditions which apply to gaining access to the healthcare delivered on Spanish territory through the relevant services assigned to such task.
If healthcare is received in another Member State, a prior evaluation shall be necessary, where so required in the National Health Service, to justify the prescribing of the healthcare benefit that has to be provided to the patient. Where such evaluation is conducted in Spain, it shall be carried out by a primary care doctor. Under no circumstances may these evaluations constitute situations of discrimination or be an obstacle to the free movement of patients, services or goods, except where objective justifications exist.
5. The reimbursement of cross-border healthcare costs shall be subject to prior authorisation in the cases laid down in Annex II.
6. Notwithstanding the foregoing, the Ministry of Health, Social Services and Equality may, following a report from the Interterritorial Council of the National Health Service, limit the application of the reimbursement rules on grounds of overriding reasons of general interest, especially as a result of planning needs aimed at ensuring a balanced range of high-quality treatments or to ensure a rational use of financial, technical and human resources. Such decision shall be restricted to whatever measures are necessary and proportional without constituting a means of arbitrary discrimination or an obstacle to the free movement of persons whose State of affiliation is Spain. Prior notice of any measures taken in this respect shall be given to the European Commission.
Article 11. Reimbursement rates which apply to patients whose Member State of affiliation is Spain who request healthcare in another Member State.
1. A transparent procedure shall be guaranteed to calculate cross-border healthcare costs which have to be reimbursed to patients whose State of affiliation is Spain according to the regulations established on public rates and prices, also taking into account the actual cost of the healthcare.
2. The public prices or rates that are applied to the delivery of healthcare services which have been approved and published by the competent health authority shall be used as the rates which apply to the reimbursement of the costs paid in other Member States by patients entitled to such healthcare in accordance with Article 10.3. Where published rates are unavailable for the provision of some kind of healthcare, these shall be set by applying objective, non-discriminatory criteria which are known in advance.
Article 12. Rates which apply to patients whose State of affiliation is another Member State who request healthcare in Spain.
Healthcare providers shall apply to patients whose State of affiliation is another Member State the same rates they apply to Spanish patients in comparable medical situations.
a) The published public prices and rates mentioned in the preceding article shall apply to the healthcare which is subject to invoicing delivered in the National Health Service's centres and services.
b) In the case of healthcare delivered by private providers, the rates these have published shall apply.
Article 13. Availability of prices and rates.
The prices and rates set forth in this chapter shall be made public by healthcare providers according to provisions set forth in Chapter III.
Article 14. Minimum requirements of the reimbursement procedure.
1. The reimbursement procedure for costs shall be laid down by the competent authorities.
2. The application for reimbursement shall be sent to the agency assigned for such purpose by the competent health authority within a maximum time limit of three months from the date payment was made for the healthcare received and shall be in keeping with the format laid down for such purpose.
3. At least the documents included in Annex I shall be attached to the application for reimbursement in order to facilitate assessing the appropriateness and amount of the reimbursement of the actual cost of the healthcare benefit.
4. Once the documentation required has been received, the competent agency shall conduct the opportune checks to determine entitlement to reimbursement, compliance with the conditions under which prior authorisation was granted, as appropriate, and the corresponding amount in accordance with the rates which apply to each case.
5. Once the processing of the procedure has finalised, a reasoned resolution shall be issued and notice thereof shall be given to the interested party, stating the appropriate complaints and appeals which may be lodged according to Act 30/1992 of 26 November.
6. The maximum time limit to serve notice of the reimbursement procedure's resolution shall be three months counting from the date the application is received by the competent health authority.
7. Once the time limit has elapsed without an express resolution being issued, the application shall be construed to have been accepted through administrative silence under the terms set forth in Article 43 of Act 30/1992 of 26 November.
CHAPTER V
Healthcare requiring prior authorisation
Article 15. Prior authorisation.
1. The delivery of cross-border healthcare for the techniques or procedures listed in Annex II shall be subject to prior authorisation by the competent authorities of the insured person's regional authority of residence, by the National Institute for Healthcare Management or by the relevant mutual society for civil servants, as appropriate, for the reimbursement of costs according to this Royal Decree.
2. A plenary session of the Interterritorial Council of the National Health Service shall issue a favourable report on the proposal for common criteria agreed upon by the Benefits, Insurance and Funding Commission to be applied by all regional authorities, the National Institute for Healthcare Management and by mutual societies for civil servants in order to grant the relevant prior authorisations provided for by this Article.
Article 16. Minimum requirements for the prior authorisation procedure.
1. The prior authorisation procedure shall be laid down by the competent health authorities. The application shall be sent to the agency assigned by the health authority and shall be in keeping with the format established for such purpose.
2. The competent health authority shall check if the conditions laid down by Article 20 of Regulation (EC) No 883/2004 of 29 April 2004 have been met. If said conditions have been met, it shall grant prior authorisation pursuant to the provisions set forth in the aforementioned Regulation, unless the patient requests application of the provisions set forth herein. In order to facilitate this choice, information shall be provided on the consequences arising from the application of the two options.
3. Once the processing of the procedure has finalised, a reasoned resolution shall be issued and notice thereof shall be given to the interested party, stating the appropriate complaints and appeals which may be lodged according to Act 30/1992 of 26 November.
4. The maximum time limit to serve notice of the resolution granting or, as appropriate, refusing prior authorisation shall be 45 days counting from the date the application is received by the competent health authority. Notwithstanding the foregoing, the competent health authority shall take into account the patient's specific disease, degree of urgency and individual circumstances when assessing an application for cross-border healthcare.
5. Once the time limit has elapsed without an express resolution being issued, the application shall be construed to have been accepted through administrative silence under the terms set forth in Article 43 of Act 30/1992 of 26 November.
Article 17. Reasons for refusing to grant prior authorisation.
The competent health authority may refuse to grant prior authorisation in the following cases:
a) Where the benefit in question is not included in the National Health Service's common portfolio of services or, as appropriate, in the corresponding regional authority's complementary portfolio or the conditions under which it is meant to be provided have not been met.
b) Where the patient will, according to a clinical evaluation, be exposed with reasonable certainty to a patient-safety risk that cannot be regarded as acceptable, taking into account evidence of the potential benefit for the patient of the sought cross-border healthcare.
c) Where the general public will be exposed with reasonable certainty to a substantial safety hazard as a result of the cross-border healthcare in question.
d) Where the benefit is provided by a healthcare provider that raises serious and specific concerns with respect of the standards and guidelines on quality of care and patient safety.
e) Where the healthcare can be provided on national territory within a time limit that can be justified on medical grounds.
In order to decide which time limit can be considered as justifiable on medical grounds, an individualised clinical evaluation shall be conducted taking into account each patient's state of health, the possible development of his disease, the degree of the his pain or the nature of his disability at the time when the request for authorisation is made.
In addition, the time limits set forth in Royal Decree 1039/2011 of 15 July establishing the framework criteria to ensure maximum time limits to gain access to the National Health Service's healthcare benefits and the rules guaranteeing current waiting times in each regional authority shall be taken into account.
CHAPTER VI
Cooperation on healthcare
Article 18. Mutual assistance and cooperation.
1. Spain shall cooperate with other Member States to facilitate the delivery of cross-border healthcare and collaborate in the areas which are set forth in this chapter.
2. Mutual assistance shall include cooperation on quality and safety standards and guidelines as well as on information transfer.
Article 19. Information transfer on health professionals.
1. For the purpose of cross-border healthcare, the competent authorities to inform the public administrations of other Member States which enquire about the right of health professionals holding a university degree or specialisation in Health Sciences to exercise their profession and on their suspension or their disqualification thereof shall be as follows:
a) The administrative agency holding responsibility for National Registry of Health Professionals governed by the tenth additional provision of Act 16/2003 of 28 May on the cohesion and quality of the National Health Service.
b) The regional authorities' departments of health within in the scope of their competencies. In this case, replies shall be sent through the Ministry of Health, Social Services and Equality, which shall coordinate the information sent.
c) The governing boards of health professional associations within the scope of their competencies, where an act sets forth the obligation of joining such associations in order to exercise professional activities or health professions. In this case, replies shall be sent through the Ministry of Health, Social Services and Equality, which shall coordinate the information sent.
2. Replies to requests for information shall be sent via the Internal Market Information System pursuant to Regulation (EU) 1024/2012 of the European Parliament and of the Council of 25 October 2012 on administrative cooperation through the Internal Market Information System and repealing Commission Decision 2008/49/EC ("the IMI Regulation").
Article 20. Recognition of prescriptions issued in another Member State.
1. Prescriptions issued for a specific patient in another Member State of industrially manufactured medicinal products for human use whose sale has been authorised by the Spanish Medicines and Medical Devices Agency or authorised according to Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 May 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency may be dispensed in accordance with the provisions set forth in Act 29/2006 of 26 July on guarantees for and rational use of medicinal products and medical devices and in Royal Decree 1718/2010 of 17 December on medical prescriptions and dispensing orders.
2. Any constraints placed on the recognition of the prescriptions set forth in the paragraph above shall be forbidden, unless such constraint is:
a) limited to what is necessary and proportionate to safeguard human health, and non-discriminatory; or
b) based on legitimate and justified doubts about the authenticity, content or comprehensibility of an individual prescription.
3. The recognition of such prescriptions shall not affect national rules governing prescribing and dispensing medicinal products, including generic or other substitution. The recognition of prescriptions shall not affect the rules on the public funding of medicinal products. The reimbursement of the costs of medicinal products included in the pharmaceutical benefits of the National Health Service shall be governed by the provisions set forth in this Royal Decree.
4. All necessary measures shall be taken, in addition to the recognition of the prescription, in order to ensure continuity of treatment where a prescription is issued in another Member State for medicinal products or medical devices available in Spain and where dispensing is sought in Spain.
5. The provisions set forth the preceding paragraphs shall not apply to medicinal products subject to special medical prescriptions.
6. The provisions set forth in this Article shall likewise apply to medical devices that are sold legally in Spain.
Article 21. European Reference Networks.
Following a prior resolution taken by the Interterritorial Council of the National Health Service at the proposal of the Committee dependant on said Council, the Ministry of Health, Social Services and Equality shall establish the corresponding procedure so that the services and units of reference of the National Health Service – assigned as such in accordance with the procedure set forth in Royal Decree 1302/2006 of 10 November establishing the bases for the procedure to assign and accredit centres, services and units of reference of the National Health Service following a report by the aforementioned Committee – may belong to or collaborate with European reference networks once the European Commission has published the instruments implementing them.
Article 22. Information on rare diseases.
1. In order to develop diagnostic and treatment capabilities for rare diseases and to broaden knowledge about them, the Ministry of Health, Social Services and Equality shall offer:
a) to health professionals: the information available on the tools existing in Spain and the European Union aimed at facilitating clinical practice for rare diseases, such as the centres, services and units of reference of the Spanish National Health Service and the European Reference Networks dedicated to these diseases;
b) to patients: information on existing tools, such as the centres, services and units of reference of the Spanish National Health Service and the European Reference Networks dedicated to these diseases.
2. Information shall be provided to patients, health professionals and those responsible for funding healthcare about the possibilities offered by Regulation (EC) No 883/2004 of 29 April 2004 to refer patients suffering from rare diseases to other Member States for diagnoses and treatments that are not available in Spain.
Article 23. European eHealth Network.
1. In order to facilitate cooperation and information transfer with other Member States, Spain shall form part of the European eHealth Network governed by Commission Decision 2011/890/EU of 22 December 2011 providing the rules for the establishment, management and the functioning of the network of national responsible authorities on eHealth.
2. The Ministry of Health, Social Services and Equality shall designate the national responsible authority on eHealth and give notice thereof to the European Commission.
3. This network shall connect the national authority thus designated to the national responsible authorities on eHealth of the different Member States.
4. The essential principles of the national responsible authority on eHealth shall be as provided in Royal Decree 4/2010 of 8 January governing the National Interoperability Framework in the area of eGovernment, which reflects the basic principles adopted by the European Interoperability Strategy and the European Interoperability Framework.
5. The national responsible authority on eHealth within the National Health Service shall oversee compliance with the European eHealth Network's objectives with respect of adherence to existing data protection and patient autonomy legislation.
Article 24. European Network for Health Technology Assessment.
1. The Ministry of Health, Social Services and Equality shall participate in the European Network for Health Technology Assessment, through which the Union will facilitate cooperation, communication and the transfer of scientific information among Member States.
2. The agencies or units of the Spanish Health Technology and Benefits Assessment Network of the National Health Service shall take part in the activities of the European network in accordance with the rules of procedure established for said European network.
3. The Council of the Spanish Health Technology and Benefits Assessment Network of the National Health Service shall assess the nature and conclusions of the work carried out within the European network and decide on the possibility of adopting and incorporating such work into the Spanish network's lines of work.
First additional provision. Application to the National Institute for Healthcare Management
Any references made in this Royal Decree to the regional authorities shall likewise apply to the Institute for Health Management (INGESA) attached to the Ministry of Health, Social Services and Equality in the circumstances which fall under its scope, as it has been assigned with healthcare competencies in the cities of Ceuta and Melilla.
Second additional provision. Relationship with other provisions.
This Royal Decree shall apply without detriment to the following provisions:
a) Royal Decree 1030/2006 of 15 September establishing the National Health Service's common portfolio of services and the procedure to update it.
b) Royal Decree 271/1990 of 23 February on the reorganisation of price intervention of pharmaceutical products for human use.
c) Royal Decree 1616/2009 of 26 October regulating active implantable medical devices, Royal Decree 1591/2009 of 16 October regulating medical devices and Royal Decree 1662/2000 of 29 September on medical devices for "in vitro" diagnosis.
d) Organic Act 15/1999 of 13 December on the protection of personal data.
e) Act 45/1999 of 29 November on the movement of workers within the framework of a cross-border service provision.
f) Act 34/2002 of 11 July on information society services and eCommerce.
g) Chapter III, Title II of Act 62/2003 of 30 December on fiscal, administrative and social measures establishing measures to apply the principle of equal treatment.
h) Royal Decree 223/2004 of 6 February governing clinical assays with medicinal products.
i) Royal Decree 1345/2007 of 11 October regulating the authorisation, registration and dispensing conditions procedure for industrially manufactured medicinal products for human use and Royal Decree 577/2013 of 26 July regulating pharmacovigilance of medicinal products for human use.
j) Royal Decree 1088/2005 of 16 September establishing the technical requirements and minimum conditions for blood donations and transfusion centres and services.
k) Council Regulation (EC) No 859/2003 of 14 May 2003 extending the provisions of Regulation (EEC) No 1408/71 and Regulation (EEC) No 574/72 to nationals of third countries who are not already covered by those provisions solely on the ground of their nationality.
l) Royal Decree 1301/2006 of 10 November establishing quality and safety standards for the donation, extraction, assessment, procedure, preservation, storage and distribution of human cells and tissue and approving the coordination and functional standards for their use in humans.
m) Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency.
n) Regulation (EC) No 883/2004 of 29 April 2004 and Regulation (EC) No 987/2009 of the European Parliament and of the Council of 16 September 2009 laying down the procedure for implementing Regulation (EC) No 883/2004 on the coordination of social security systems.
n.bis) Royal Decree 1837/2008 of 8 November transposing into the Spanish legal system Directive 2005/36/EC of the European Parliament and of the Council of 7 September 2005 and Directive 2006/100/EC of the Council of 20 November 2006 on the recognition of professional qualifications, as well as on certain aspects of exercising the profession of lawyer.
o) Regulation (EC) No 1082/2006 of the European Parliament and of the Council of 5 July 2006 on a European grouping of territorial cooperation (EGTC).
p) Regulation (EC) No 1338/2008 of the European Parliament and of the Council of 16 December 2008 on Community statistics on public health and health and safety at work.
q) Regulation (EC) No 593/2008 of the European Parliament and of the Council of 17 June 2008 on the law applicable to contractual obligations (Rome I), Regulation (EC) No 864/2007 of the European Parliament and of the Council of 11 July 2007 on the law applicable to non-contractual obligations (Rome II) and other Union rules on private international law, in particular rules related to court jurisdiction and the applicable law.
r) Royal Decree 1723/2012 of 28 December regulating the activities of the extraction, clinical use and territorial coordination of human organs destined to transplants and establishing quality and safety requirements.
s) Regulation (EU) No 1231/2010 of the European Parliament and of the Council of 24 November 2010 extending Regulation (EC) No 883/2004 and Regulation (EC) No 987/2009 to nationals of third countries who are not already covered by these Regulations solely on the ground of their nationality.
Third additional provision. Cross-border healthcare in special regimes for civil servants.
1. The regional authorities and INGESA shall be the competent administrations in procedures involving cross-border healthcare of persons who are entitled to or beneficiaries of special social security regimes managed by mutual societies for civil servants that have joined their respective healthcare services through the established procedure.
2. As regards the members of such mutual societies who have opted to receive healthcare through other methods, the relevant mutual society, as the competent administration, shall set forth in specific legal instruments the way and management procedure to ensure effective entitlement to cross-border healthcare under the terms stipulated herein, as well as the rates which apply to reimbursement.
Fourth additional provision. Information to be provided to the European Union.
1. The Ministry of Health, Social Services and Equality shall provide the necessary assistance to the European Commission and provide it with any available information to draw up the reports for which said institution holds responsibility according to Union law, while respecting the security guarantees on the processing of personal data set forth in Spanish legislation.
2. For the purpose of fulfilling the obligation set forth in the preceding paragraph, the regional health services, the Institute for Healthcare Management and the different mutual societies for civil servants shall provide said ministerial department with the necessary information, particularly information on patient flows, financial figures on patient mobility, prior authorisation and other aspects connected to the reimbursement of cross-border healthcare costs.
Fifth additional provision. Analysis and evaluation of this Royal Decree's application and bringing it up to date.
1. The Interterritorial Council of the National Health Service shall, through the Benefits, Insurance and Funding Committee, analyse during the first year after the entry into force of this Royal Decree and subsequently with the periodicity that is determined patient flows, financial and organisational aspects of patient mobility, the application of reimbursement and prior authorisation procedures and the kinds of benefits requested in cross-border healthcare, and shall assess the evolution of these factors in order to introduce elements to enhance guarantees on patients' rights within the framework of this Royal Decree.
2. Said Benefits, Insurance and Funding Committee shall also analyse the kinds of benefits subject to prior authorisation and, on the basis of the outcome of its analysis, the benefits included in Annex II shall be brought up to date under the terms provided in the fourth final provision.
Sixth additional provision. No increase in public expenditure.
The measures included in this piece of legislation may not involve an increase in allocations or remuneration, or in any other personnel expenses.
Seventh additional provision. Application to the European Economic Area.
All the provisions contained in this Royal Decree on cross-border healthcare in European Union Member States shall also be construed to apply to the States which form part of the Agreement on the European Economic Area once the corresponding legal instruments have been adopted.
Single transitional provision. Coexistence of prescription and dispensing orders.
As from the entry into force of this Royal Decree, medical prescriptions and hospital dispensing orders according to the provisions of this Royal Decree may coexist with those used at the moment of its publication during a period of 12 months. Once said period has elapsed, only medical prescriptions and hospital dispensing orders that are in keeping with this legislation shall be valid.
Nonetheless, any medical prescriptions issued for dispensing in another Member State of the EU shall be in accordance with the provisions set forth in this Royal Decree from the moment it enters into force.
First final provision. Amendment of Royal Decree 1718/2010 of 17 December on medical prescriptions and dispensing orders.
Royal Decree 1718/2010 of 17 December shall be amended as follows:
-
I.- Article 3 shall be worded as follows:
"Article 3. Common medical prescription formats and data.
1. Public or private medical prescriptions may be issued on hard copy to be completed by hand or by computer and on electronic media. They shall be supplemented with a patient information sheet, which must be handed over to the patient, containing information on the treatment needed in order to facilitate the proper use of the medicinal products or medical devices prescribed.
2. The prescriber shall include in the patient information sheet the basic mandatory data which are essential for the medical prescription's validity, as indicated below:
a) The patient's data:
1.- Name, two surnames and date of birth.
2.- In medical prescriptions issued by the public health system, the patient's personal identification code, as reflected in his individual health card and assigned by his health service or by the competent administrations of the special health regimes. In the case of foreign nationals who do not have the aforementioned card, the code assigned to their European Health Card or their Provisional Replacement Certificate (PRC), or the corresponding European form stating entitlement to healthcare, or the passport number of foreign nationals from countries outside the Community. In any event, the patient's contribution regime shall also be included.
3.- In medical prescriptions issued by the private health sector, the patient's identity card number or tax information number. Should the patient not have such documentation, the identity card number or the tax identification number of one of their parents shall be included in the case of children or, as appropriate, of their legal representative, and the passport number in the case of foreign nationals.
b) Data on the medicinal product:
1.- Name of the active principle(s).
2.- Brand name of the medicinal product if it is a biological medicinal product or the health professional prescribing it deems it necessary from a medical standpoint according to the provisions set forth in the Act 29/2006 of 26 July on guarantees for and rational use of medicinal products and medical devices. In such case, the use of the brand name shall be briefly justified.
3.- Dosage and pharmaceutical form and, where appropriate, a mention of the recipients: infants, children, adults.
4.- Way or form of administration, should it be necessary.
5.- Format: number of units per package or contents thereof by weight or volume.
6.- Number of packages or specific number of units of the medicinal product to be dispensed.
7.- Posology: number of units to be administered per intake, frequency of intakes (by day, week, month) and total duration of treatment.
The data referred to in items 5 and 6 shall only be mandatory in medical prescriptions issued on hard copy. In medical prescriptions issued on electronic media, the prescriber shall only have to include them where the electronic system does not generate them automatically.
c) Prescriber's data:
1.- Name and two surnames.
2.- Direct contact details (e-mail and telephone number or fax, including international dialling code).
3.- Professional address, including town and the wording "Spain". Reference to public establishments, institutions or bodies may only be included in official medical prescriptions issued by them.
4.- Professional qualification.
5.- License number or, in the case of National Health Service medical prescriptions, the identification code assigned by the competent administrations and, as appropriate, the health professional's officially accredited specialisation.
In medical prescriptions of the armed forces' Military Health Network, the number of the medical practitioner's military ID card may be used instead of the license number. The health professional's specialisation shall, as appropriate, be indicated.
6.- The signature shall be personally written out once the mandatory data have been included and the prescription completed. An electronic signature shall be required for ePrescriptions, which shall be signed according to the criteria laid down by Act 11/2007 of 22 June on citizens' electronic access to public services.
In prescriptions of the National Health Service, the prescriber's data referred to in items 3 and 5 may additionally be included in a way that allows for the mechanisation of such data by the health services and mutual societies for civil servants.
d) Other data:
1.- The date of prescription (day, month and year): date on which the prescription is made out.
2.- The expected date of dispensing (day, month and year): date from which the prescription may be dispensed in the case of successive instances of dispensing for chronic treatments or medicinal products whose dispensing is renewable.
3.- Order number: number indicating the prescription's dispensing order in the case of successive instances of dispensing for chronic treatments or medicinal products whose dispensing is renewable.
The data referred to in items 2 and 3 shall only be mandatory in medical prescriptions on hard copy.
In addition to the data set out in the items above, the health authorities' approval, if appropriate, shall be included according to Royal Decree 618/2007 of 11 May regulating the procedure to establish specific reservations on prescription and dispensing conditions of medicinal products by means of approvals. In the case of ePrescriptions, approval shall be granted in the manner set forth in Article 8.7 of this Royal Decree.
In medical prescriptions on hard copy and in the patient information sheet in the case of ePrescriptions, a clause informing the patient about the terms laid down by Organic Act 15/1999 of December 13 on the protection of personal data.
3. The patient information sheet shall be differentiated from the prescription and may be separable from it or may be on a different form where the prescriber can list all the medicinal products and medical devices prescribed, thereby providing the patient with information on the entire treatment and diagnosis, if appropriate in the prescriber's judgement.
4. All the data and instructions contained in the medical prescription shall be clearly readable, without prejudice to possibly being encoded with optical characters. Mandatory data in medical prescriptions may not have any changes or deletions, unless these are signed once again by the prescriber."
-
II.- Article 5.1 shall be worded as follows:
"1. Official medical prescriptions used for the National Health Service's pharmaceutical benefits shall be prescribed by authorised health professionals exercising their functions within the scope of the National Health Service. Said prescriptions shall comply with the provisions set forth in this Royal Decree, with the details contained in this Chapter and the requirements established by the competent health authorities within the scope of their competencies.
Official prescriptions shall be in keeping with the following basic differentiation criteria in accordance with classification abbreviations or codes in the individual health card database, which shall be printed as alphanumeric characters or codes in the upper right of the prescriptions as follows:
a) TSI 001 code for users exempted from contributing.
b) TSI 002 code for users with a reduced contribution of 10%.
c) TSI 003 code for users with a contribution of 40%.
d) TSI 004 code for users with a contribution of 50%.
e) TSI 005 code for users with a contribution of 60%.
f) TSI 006 code for users belonging to mutual societies for civil servants with a contribution of 30%.
g) ATEP for prescriptions for occupational accidents or diseases.
h) NOFIN for prescriptions of medicinal products and medical devices which are not funded.
i) DAST for prescriptions of medicinal products and medical devices prescribed to users under the application framework of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients' rights in cross-border healthcare. Users have to pay the full amount."
-
III.- A new article 15.bis is added, which shall be worded as follows:
"Article 15 bis. Dispensing of prescriptions issued in another Member State of the European Union.
1. Prescriptions issued for a specific patient in another Member State of industrially manufactured medicinal products for human use whose sale has been authorised by the Spanish Medicines and Medical Devices Agency or authorised according to Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 May 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency may be dispensed in accordance with the provisions set forth in Act 29/2006 of 26 July on guarantees for and rational use of medicinal products and medical devices in the prescription and dispensing of medicinal products.
2. For this purpose, the elements that shall at least be included in prescription are indicated below:
a) Patient identification: surname(s), name (written out in full, not only initials) and date of birth
b) Authentication of the prescription: date of issuance
c) Identification of the health professional prescriber: surname(s), name (written out in full, not only initials), professional qualifications, direct contact details (e-mail and telephone number or fax with the international dialling code), professional address (and Member State), and signature (written or digital, depending on the media chosen to issue the prescription)
d) Identification of the medicinal product or medical device: common name as defined in Article 1 of Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use; brand name if it is a biological medicinal product or the health professional prescribing it considers it necessary from a medical standpoint and, in such case, the use of the brand name shall be briefly justified on the prescription; pharmaceutical form; quantity; dosage, as defined in Article 1 of Directive 2001/83/EC of 6 November 2001; and posology guideline.
3. The recognition of a prescription shall not affect the pharmacist's right to refuse dispensation of a medicinal product prescribed in another Member State on ethical grounds or where reasonable doubts arise about the authenticity or validity of the prescription submitted, unless the prescription's validity can be verified.
4. The provisions set forth the preceding paragraphs shall not apply to medicinal products subject to special medical prescriptions.
5. The provisions set forth in this Article shall likewise apply to medical devices that are legally sold in Spain."
-
IV.- The Annex is replaced, which shall henceforth be worded as follows:
"ANNEX
Basic criteria for medical prescriptions and dispensing orders
1.- Official medical prescriptions of the National Health Service and mutual societies for civil servants.
Official medical prescriptions that are to be used for the pharmaceutical benefits provided by the National Health Service, including mutual societies for civil servants, prescriptions for medicinal products and medical devices when applying cross-border healthcare and prescriptions for non-funded medicinal products, shall be in keeping with the common characteristics laid down in this Annex.
1. The name of the competent authority or agency issuing the prescription shall be indicated at the top left-hand corner of the forms included in this Annex and "National Health Service" shall be included at the top right-hand corner.
The dimensions of the spaces dedicated to each datum which should be included in the prescription shall be in keeping with the publishing or printing requirements the health authorities may establish.
2. Technical specifications of medical prescription forms.
a) Approximate dimensions: 22 x 12 centimetres.
b) Official prescriptions shall be in keeping with the following basic differentiation criteria:
As set forth in item 1 above, the name of the management entity or agency issuing the prescription shall be included in the space reserved for the competent authority or agency.
The initials or classification code in the individual health card database shall be included in the space reserved for "Contingency". These shall be printed alphanumerically or encoded according to the following scheme:
a) TSI 001 code for users exempted from contributing.
b) TSI 002 code for users with a contribution of 10%.
c) TSI 003 code for users with a contribution of 40%.
d) TSI 004 code for users with a contribution of 50%.
e) TSI 005 code for users with a contribution of 60%.
f) TSI 006 code for users belonging to mutual societies for civil servants with a contribution of 30%.
g) ATEP for prescriptions for occupational accidents or diseases.
h) NOFIN for prescriptions of medicinal products and medical devices which are not funded.
i) DAST for prescriptions of medicinal products and medical devices prescribed to users under the application framework of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients' rights in cross-border healthcare. Users have to pay the full amount.
The status of the mutual society member shall be included in the space reserved for "Regime of use" in the prescriptions of mutual societies for civil servants.
The space "Prescription code" shall be reserved for the prescription's identification code.
The space "Information for the pharmacist and approval as appropriate" shall be reserved for the information the prescriber considers necessary to provide the pharmacist and should approval for its dispensation be required.
c) The name and address of the company shall additionally be indicated after the data on the prescriber and the date of prescription in prescriptions for occupational accidents or diseases.
d) In prescriptions for medicinal products that are not funded and in prescriptions of medicinal products and medical devices prescribed to users in application of the cross-border healthcare, the wording "Not valid for invoicing" shall be included. Spaces reserved for tear-out labels or similar shall not be included in the document and such spaces shall be used to print the aforementioned wording.
e) Coupon books shall be made according to the requirements established by the health services and the competent administrations of the mutual societies for civil servants.
f) Patient information sheet: approximate dimensions of 22 x 12 centimetres or DIN A-4 size, and may be adapted to the size deemed most appropriate to provide the patient with information on his treatment.
3. Active medication and patient information sheet for the ePrescription system.
a) Approximate dimensions: DIN A-4 size.
b) As set forth in item 1 above, the name of the management entity or agency which issues the prescription shall be included. Regarding the data to be included:
The initials or classification code in the individual health card or mutual society member database shall be included in the space reserved for "Regime of use" in prescriptions issued by mutual societies for civil servants.
"Prescription code": a space reserved for the unique and unrepeatable piece of data assigned by the electronic system to identify the prescription of each medicinal product or medical device.
c) These shall be created according to the requirements established by the health services and the competent administrations of the mutual societies for civil servants.
2.- Medical prescriptions for private healthcare.
The technical specifications of medical prescriptions used for healthcare delivered outside hospitals and which are not official National Health Service medical prescriptions, including prescriptions of mutual societies for civil servants, shall fulfil the provisions set forth in item 1 of this Annex, with the relevant exceptions such as the spaces reserved for "Regime of use" and "Tear-out labels or similar", which shall not be included in the document.
"Medical prescription for private healthcare" shall be indicated in the space reserved for "Contingency".
Numbering elements and identification of medical prescriptions may be included at the top left-hand corner.
The name of the institution, if appropriate, may be indicated at the top left-hand corner.
3.- Hospital dispensing orders.
Hospital dispensing orders used in hospitals for outpatients shall be in keeping with the provisions laid down by this Annex for medical prescriptions with the relevant exceptions, such as the spaces reserved for tear-out labels or similar, which shall not be included in the document, and shall allow several medicinal products to be prescribed.
The wording "Hospital dispensing order" shall be included in the space reserved for "Regime of use" and, in the National Health Service, the abbreviation or classification code in the individual health card database shall be included in the space reserved for "Contingency", which shall be printed alphanumerically or encoded according to the scheme set forth in item 1 of this Annex.
Furthermore, the hospital dispensing order shall specify the pharmacy service instead of the pharmacy and also include the medical record number and the medical service or clinical unit in the patient's data, along with the prescriber.
The date of the patient's acknowledgement of receipt and his signature shall serve as proof of dispensing.
Private hospitals and the competent authorities and agencies may, should they deem it appropriate, include references or data and the necessary number of copies in hospital dispensing orders to ensure proper use and control thereof.
4. - Dispensing orders.
In general terms, the dispensing orders referred to in Article 1, item c) of this Royal Decree shall be subject to the basic criteria laid down in this Annex for medical prescriptions and shall be in keeping with the characteristics described in the corresponding form.
More specifically, official dispensing orders of the National Health Service, including those of mutual societies for civil servants, shall be subject to the basic criteria established in item 1, while dispensing orders for private healthcare shall adhere to the basic criteria set forth in item 2."
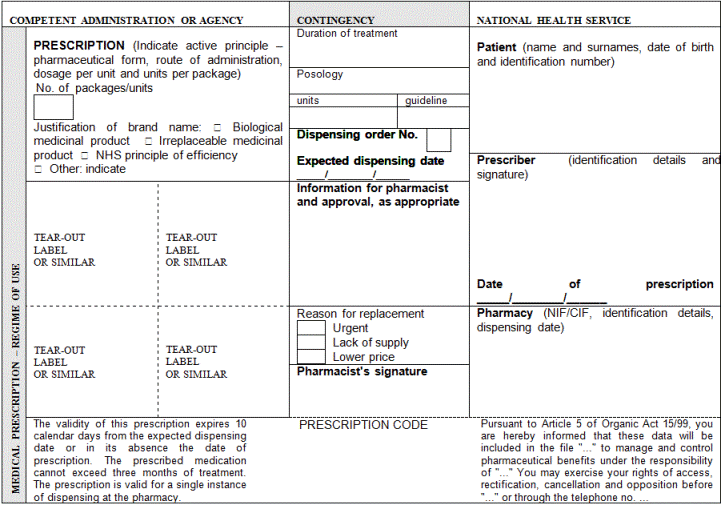
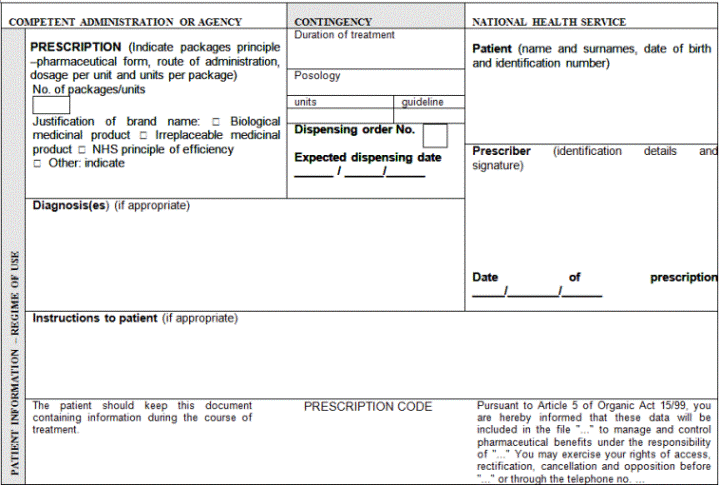
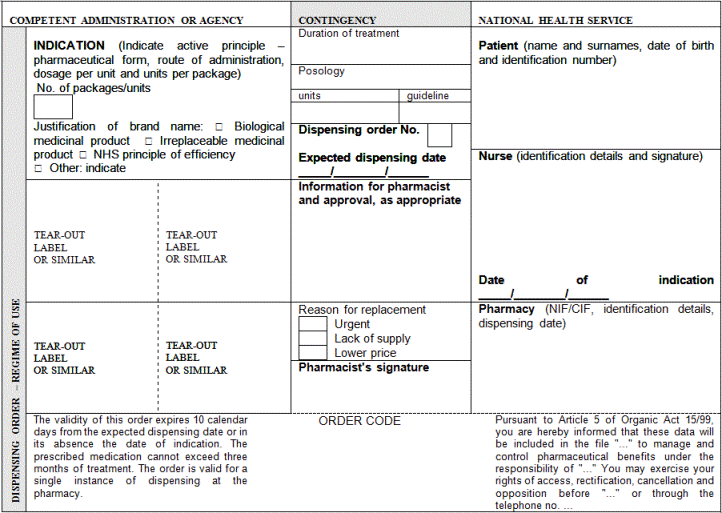
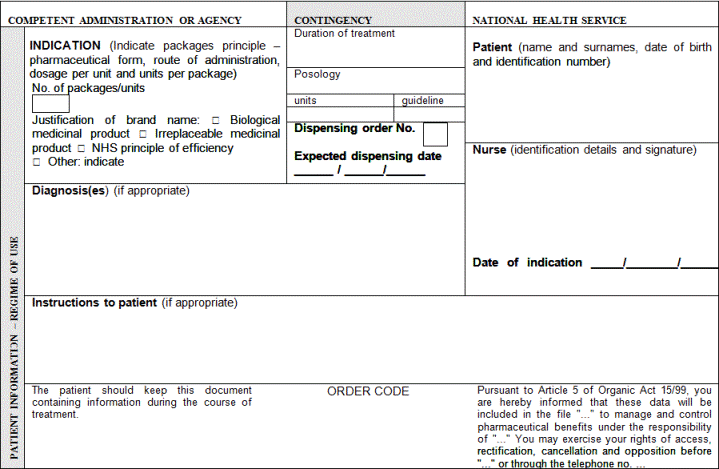
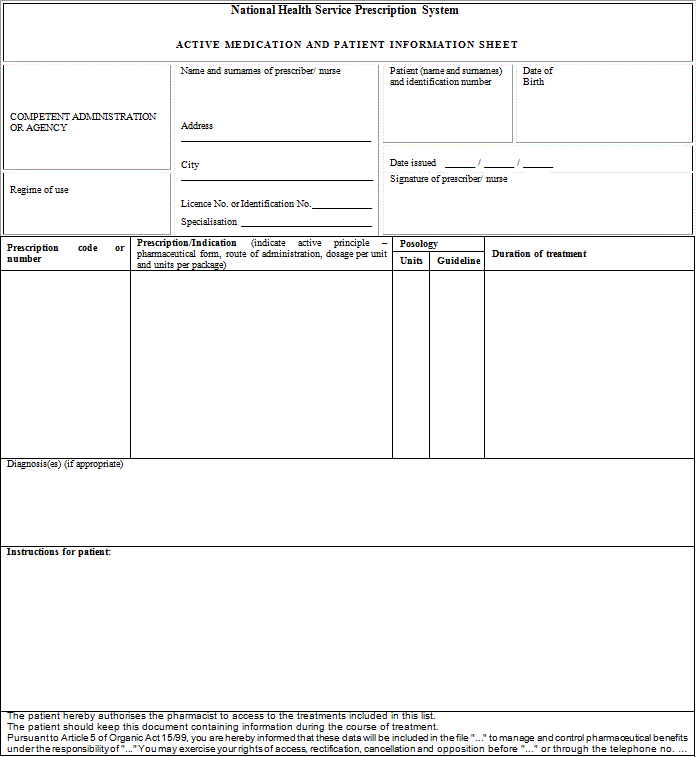
Second final provision. Attribution of competence.
This Royal Decree is enacted pursuant to the provisions set forth in Article 149.1(16) of the Constitution, which grants the State exclusive competence on the general rules and coordination of health. The amendment of Articles 3 and 5 and the annex of Royal Decree 1718/2010 of 17 December on medical prescriptions and dispensing orders, which is done through the first final provision, is excluded from the above and grounded on the State's competence to enact legislation on pharmaceutical products.
Third final provision. Transposition of European Union Law.
Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients' rights in cross-border healthcare and Implementing Directive 2012/52/EU of the Commission of 20 December 2012 laying down measures to facilitate the recognition of medical prescriptions issued in another Member State are transposed into the Spanish legal system through this Royal Decree.
Fourth final provision. Implementing provisions and updating of annexes.
The Minister of Health, Social Services and Equality is hereby empowered to issue the necessary provisions to apply and implement this Royal Decree, as well as to amend its annexes following a report from the Interterritorial Council of the National Health Service in order to adapt them to advances in scientific and technical knowledge or to adapt them to European Union legislation.
Fifth final provision. Entry into force.
This Royal Decree shall enter into force on the date following that of its publication in the "Official Journal of the State" (Boletín Oficial del Estado).
Done in Madrid on 7 February 2014.
JUAN CARLOS, King
The Minister of Health, Social Services and Equality,
ANA MATO ADROVER
ANNEX I
Documentation for the reimbursement procedure
1. Original invoices from the healthcare provider or dispensing establishment, payment of which shall be accredited by the issuer. At least the following shall be indicated:
a) Patient identification: name, surname(s) and DNI, NIE or passport number.
b) Identification of the natural or legal person issuing the invoices: name or trade name and address.
c) Name of the clinical service or unit.
d) Identification data of the professional responsible for cross-border healthcare.
e) Detailed breakdown of the different healthcare items in the manner specified by competent health authority, the amount of each and the date they were performed.
f) As appropriate, the name of the medicinal product, medical device or dietetic food for special medical uses dispensed, the number of packages dispensed, the amount paid by the patient and the date of dispensing.
2. Copy of the medical prescription or report, which shall include the following:
a) Clinical reason for providing cross-border healthcare.
b) Diagnostic procedures or main and secondary therapeutic procedures performed in cross-border healthcare (indicating, where possible, an approved identification code, such as ICD9-CM or similar).
c) Follow-ups that have to be carried out and their estimated time.
d) Any other data deemed necessary to highlight in order to clarify the healthcare received or its actual cost, provided such data is strictly necessary to assess the appropriateness or amount of the reimbursement.
ANNEX II
Health benefits subject to prior authorisation
The following shall be subject to prior authorisation:
1. Any kind of healthcare involving hospital accommodation of the patient for at least one night.
2. Regardless of item 1, any techniques, technologies or procedures included in the National Health Service's common portfolio of services which have been selected because they require the use of highly specialised medical procedures or equipment, the need to provide care to patients suffering complex problems or due to their cost-intensiveness, including:
a) Positron Emission Tomography (PET) and in combination with CT (PET-CT) and SPECT.
b) Assisted human reproduction.
c) Dialysis.
d) Major outpatient surgery requiring the use of a surgical implant.
e) Radiotherapy treatments.
f) Pharmacological treatments or treatments with biological products the monthly cost of which exceeds €1,500.
g) Radiosurgery.
h) Genetic testing aimed at diagnosing complex cases, including prenatal and preimplantation diagnoses, presymptomatic genetic and carrier testing, pharmacogenetic testing and pharmacogenomics.
i) Treatments of disabilities which require the following for their correction or improvement: electric wheelchairs, upper limb prostheses except partial hand prostheses, lower limb prostheses except partial foot prostheses, hearing aids and leg braces.
j) Treatments involving complete formulae for home enteral nutrition, and formulae and modules for congenital carbohydrate, amino acid and lipid metabolism disorders.
k) Healthcare for pathologies and performance of procedures for which services of reference have been designated in accordance with Royal Decree 1302/2006 of 10 November establishing the rules for the procedure to designate and accredit the National Health Service's centres, services and units of reference included in Annex III of Royal Decree 1207/2006 of 20 October governing the management of the Healthcare Cohesion Fund, or which have been designated as services of reference in Europe.
If you want to find any information related to this website, please use the search application on the top of the page